The next generation of proteins
Replacing a 100 year old method
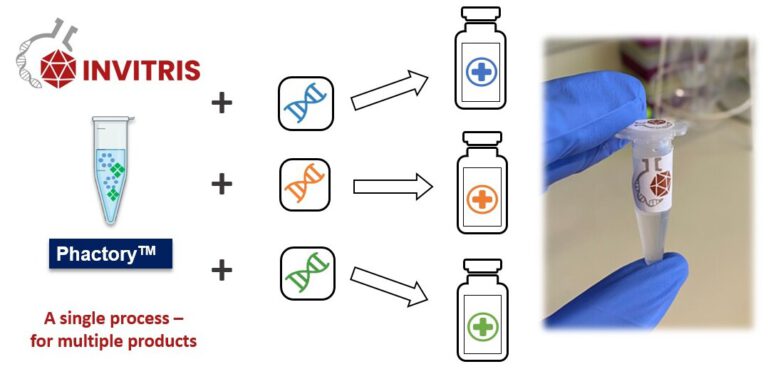
One Platform to conquer all.
We partner with other companies to: A) create new products and B) manufacture them.
Our universal platform technology enables generating synthetic proteins at the highest yields and purity. This platform is based on cell-free expression and allows creating entirely novel proteins with entire novel mechanisms of action and manufacture them at scale.
While our primary focus is on human therapeutics, we have worked on industrial use-cases too. We currently employ prokaryotic cell-free expression systems and are advancing our technology to eukaryotic cell-free expression systems.
Our specialities are: phages, tailocins, endolysins, neoantigens, vaccines, antibodies, and toxic proteins (e.g., nucleases and proteases).
Our industries are: human health, agriculture, animal health, food, and industrial chemistry.


Supported by

